The Age of New Medicines: How AI is Empowering & Disrupting the Discovery of Proteins, Biologics, Therapeutics & Materials
/Interview with Dr. Molly Gibson, Co-Founder and Chief Strategy & Innovation Officer at Generate:Biomedicines, Origination Partner at Flagship Pioneering
Dr. Molly Gibson, Co-Founder and Chief Strategy and Innovation Officer at Generate:Biomedicines.
At Flagship Pioneering, Dr. Gibson operates as part of a venture-creation team to found and grow companies at the intersection of biology and machine learning including Generate:Biomedicines, Tessera Therapeutics, and Cobalt Biomedicines (merged into Sana Biotechnology).
Before joining Flagship in 2017, she led computational biology at Kaleido Biosciences.
She currently serves as Co-Founder and Chief Strategy and Innovation Officer at Generate:Biomedicines, a new kind of therapeutics company existing at the intersection of biology, machine learning and biological engineering. The company is dedicated to revolutionizing drug discovery within the protein therapeutics space. Rather than relying on traditional approaches that involve discovering new molecules through natural processes and evolution, they employ algorithms to understand the rules governing proteins and their functionality. This allows the team to generate entirely novel molecules tailored to specific requirements, paving the way for creating more effective, cost-efficient and safer drugs.
LDV Capital's Founder & General Partner Evan Nisselson discussed with Molly how AI and machine learning empower and disrupt the scientific process across life sciences, biotech, advanced materials and more. This will have a tremendous impact on business and society.
If you missed our 10th Annual LDV Vision Summit, this is your chance to watch the video or read our shortened & lightly edited transcript below. Check out speakers and themes for our 11th Annual LDV Vision Summit and join us on Marh 25, 2025!
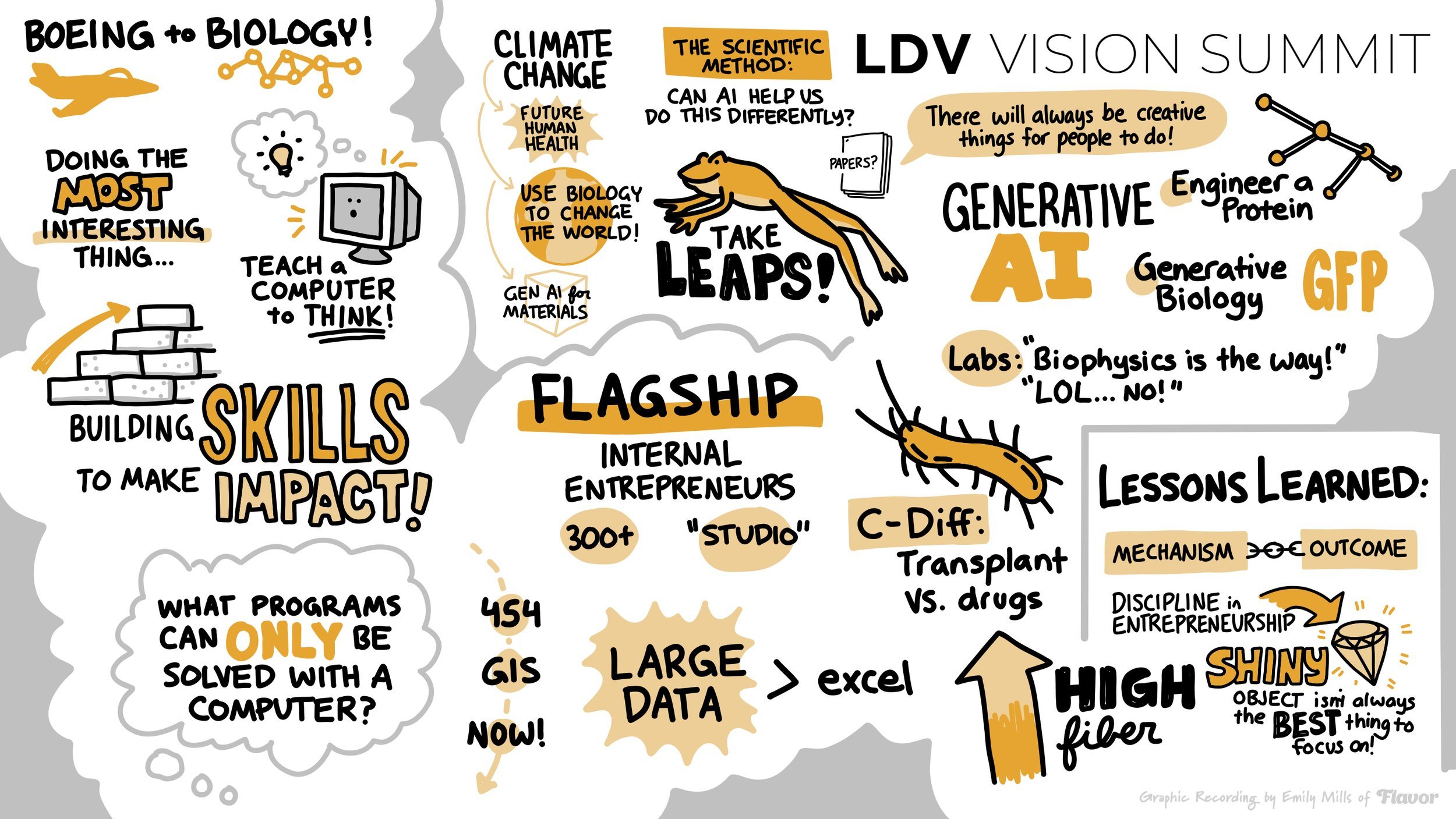
Evan: You started as a computer scientist and software engineer at Boeing and built flight simulators for the F-15. How did you go from that to biology and what did you love about that? Why did you love or why do you love biology more?
Molly: This is an interesting path and it is a theme throughout my career: doing the most interesting thing at any moment in time. In undergrad, I was not one of the people who went to school like, "I know exactly what I want to do. I'm going to be a dentist." Or, "I'm going to be a doctor, or I have a defined career path." I was exploring and trying to figure out what I wanted to do. I took a lot of different classes. I took biology classes, but I fell in love with computer science and the idea that you could teach a computer to think. We'll get into the actual teaching it to think, like in the generative AI sense, but this was the logic, just being able to program, even just for loops.
I fell in love with computer science and programming and software engineering, and so did my degree in that, but along the way, I took a lot of classes at a liberal arts school in biology and science. After my degree, I got approached by Boeing for a role there, and it just seemed interesting. At the moment, being able to apply what I had learned to something that was important in the world and in some instances could feel like you were playing a game every day when you went to work.
As I started the job, I realized that it wasn't fulfilling some of the things that I wanted the most in my career. One, applying my expertise or applying my skills to solving the most important problems that humans face today. And two, the creativity around, how computers solve challenges that aren't straightforward. These aren't physics problems or engineering problems. What drew me to biology was thinking that biology is complicated. There are no equations to describe it. How do we use computers to solve problems in life sciences? That was why I started there and why I quickly pivoted away.
Evan: We have a lot of Ph.D’s in the audience and some of them are probably thinking about commercializing their research…What topics did you choose for your research, and did you consider the possibility of commercializing any of them at that time?
Molly: When I started my PhD, I never thought I would be an academic. I wasn't thinking of going into academia. And so, I wasn't setting myself up for what's the best postdoc. I was setting myself up to have built the skill set that would be required to make an impact. I don't even think I knew what an entrepreneur was at the time, to be honest. I just had this intuition that I wanted to build things, I wanted to build companies, and I didn't have words for it.
Evan: Did you see other people building things?
Molly: I grew up in a remote area in central Iowa. I wasn't surrounded by entrepreneurs. I went to WashU in St. Louis. I did my PhD and I tell a little bit about that wonderful school, and amazing people, and it's why I went there, but I wouldn't say that they drove an entrepreneurial culture. Now my comparisons are places like MIT. It's an unfair comparison, but I didn't see that. I remember having a conversation towards the end of my PhD with a professor and describing what I wanted to do and they kind of looked at me with this blank stare like, "How are you going to do that?". I didn't know how to do that, but I knew I had to get into the ecosystem.
Dr. Molly Gibson, Co-Founder and Chief Strategy and Innovation Officer at Generate Biomedicines, Origination Partner at Flagship Pioneering, and LDV Capital’s Evan Nisselson
I was looking for two things:
I wanted to join a lab where the PI was supportive and drove growth in me as an individual. People were really important to me. That was probably my #1 decision. I would pick people over content, people over topic every day of the week.
I wanted to work on problems that wouldn't be able to be solved without a computer. It wasn't something that you could do another way.
That's why I started focusing on what is the microbiome. It's all of the bacteria that live inside of us, on us, on our skin, in our environment, and on every surface, around us. There are these small communities of organisms that live everywhere and they affect our environment, they affect our health, and they affect disease. People have gotten familiar with viruses from the pandemic, but this is something that is living inside of us.
Our gut is full of bacteria and the bacteria do important things. They break down our foods, they regulate our immune system, and they regulate our immune response. They're sensing things for us, but to understand these communities, you have to have computers, because they're complex, and they're dynamic. You have to be able to analyze large data sets. That's what I focused on, and at the time I didn't know if that was even commercializable, to be honest.
Evan: What was the definition of large data back then and what is it now? It's got to be exponentially different.
Molly: It's totally different! This was at the beginning of even next-generation sequencing. If people are familiar with sequencing technologies, like 454 sequencing to what the Illumina technology looks like today in NGS. The scale of data, even in sequencing was changing throughout the time I was even in my PhD. When people think about large data today, they're talking about all of the internet.
I would say when I was thinking about large data then, it was something you couldn't analyze in Excel. That was kind of the distinction at that time in which you needed a computer to process the data, and this was orders of magnitude beyond what you could process in Excel, but still not anywhere close to what we think about in the foundation model world today. Definitely not in general AI, but also in foundation models and biology that you're starting to see.
Evan: How and why did you join Flagship Pioneering, which founded Moderna?
Molly: I joined not Flagship, but one of the companies that they spun out. Let me describe Flagship a little bit. We look like a venture capital firm. We have funds like a venture capital firm would, but the operations of the organization look dramatically different. We don't invest in external ventures. We have internal entrepreneurs who are building and growing companies that our fund invests in, and we have all the support systems to do that. We have over 500 people, which is not a traditional VC structure as you know.
Evan: It's more of a studio structure, with a focus on life sciences, biotech and materials. One of the benefits, obviously, is it takes much longer probably to create advancements in tech versus a software business that doesn't relate to life sciences.
Molly: Much more time and much more capital. It requires a unique perspective on value creation. That's one of the things that Flagship and the institution have done well is figure out how to create value from the types of innovations you can have in biotech. Those aren't just assets, they're also platforms. And how do you create products off of platforms?
When I was done with my PhD in around 2015, I had done all my research in the microbiome space, and this was about when we were starting to understand the benefit of fecal microbiome transplants for C. diff. This is an incredibly difficult bacterial infection of the gut that causes immense pain in patients and is constantly recurring. Even when it's treated, it comes back. When it comes back, what they realize is that oftentimes we try to treat the organ by sometimes just antibiotics, but what people are realizing is that when it's diseased and you think about the gut microbiome as an organ in this context, when it's diseased, you have to replace it, so people would start to replace it with someone else's, and that was done through transferring feces from one human to the other human in their gut. Flagship was working on companies like Sirius Therapeutics, which is one of the companies I got connected with someone who has an approved drug in this space to create these types of treatments that didn't have to be sourced from the feces of others but could be grown and incubated in the lab, which made it a much more reproducible and systematic therapy. They were doing that type of research in the microbiome space, but they're also starting to think about other innovations that could be done in the microbiome space. That’s where I got connected with Kaleido Biosciences, and the focus was on how to use fiber or novel glycans that we can synthesize. Instead of using something like a fecal transplant to wipe out and replace the gut microbiome, how can we modulate it with everyday elements, like fiber?
Molly is speaking with one of her fellow co-founders at Generate:Biomedicines, Gevorg Grigoryan. Photo courtesy of Flagship Pioneering.
Evan: It is so obvious and it makes sense now, but years ago without computers, this wasn't possible, right?
Molly: Exactly! There's so much research out there for diabetes, obesity, and all types of metabolic disease that people with high-fiber diets did better, but there was no control over the actual molecule of what fiber it was. It could be many different types of molecules. There's no real understanding of the science behind it and the connection between the actual drug, in this case, fiber, to what was happening.
Kaleido hypothesized that, just like with any small molecule where you use structure-guided design in response to how it affects human biology, you could apply the same approach to glycans or fibers. That's what we were doing, which was different and interesting. It was a fun place to start and it got me connected to the ecosystem. I learned what Flagship was. I learned entrepreneurship.
I was a scientist at Kaleido, and I learned the importance of being able to truly connect the underlying mechanism of a drug to its outcome and its impact. All of my future work has been focused on places where we have true underlying mechanisms associated with what we're treating and we can connect those to large and impactful problems in the world in ways that maybe we haven't been able to do with other fields.
Evan: I was an entrepreneur for 18 years and sometimes I felt like, "Wow, this is a huge problem. We got to solve it." But years later I realized, "Well, that was interesting, but it wasn't a morphine kind of problem. It was a vitamin or an aspirin – these are important, but not huge." How do you know it's a big enough problem or there's a real enough solution?
Molly: This is where discipline and entrepreneurship are important because oftentimes entrepreneurs are by nature, creative, driven people and want to solve problems. We get intrigued and people call it “the shiny ball object”. One of the challenges is that the new shiny thing might not always be the most important thing for you to be working on. While you can make a case for it, I can make a case for lots of technology and the need for them. It's always a question of opportunity costs in my mind.
It's always a question of, "If you did that, what are you not going to do?"
Even when I was starting to think about what I wanted the first company that I founded to look like, I had many ideas, some of which I had pitched internally, and I am sure if I had pushed, I could have gotten funded, but I sat there after pitching on one of them where I was like, "All right, I can get this funded. They'll support me in this. Do I want to spend the next five years of my life solving this problem?"
Evan: And sometimes it's not five but up to ten and twelve!
Molly: Exactly! I couldn't say like, "Oh yes, I am excited. I want to solve this problem! In five years, I would be happy if I was still working on it." And so, I passed up on that idea and I kept looking and that's where I found Generate:Biomedicines.
Evan: What's great is that there are a lot of synergies here, even though we operate in different markets. LDV Capital has been investing in generative AI since 2018 before that term existed. We've got 9 companies and you co-founded Generate:Biomedicines in 2018, leveraging generative AI for drug discovery and development in the same year before that term existed. To clarify, there were GANs and other technical terms that different industries used. Tell us about Generate:Biomedicines and what was your vision for that one and what's being done now.
Molly: As you mentioned, we first started working on this before the term “generative AI” existed. When I was talking to some very respected machine learning scientists, it was before many people believed generative AI would be a thing, and I think this was partially because of how challenging GANs were to get to work, but other reasons as well.
There was a problem that a colleague was working on and they were talking about how hard it was to engineer a protein. I didn't know anything about proteins. I barely understood what a protein structure was, but they kept telling me, "If only I could get this protein that does this to do this." I started asking them about the problem and trying to understand, "Well, what evidence do you even have that it can do that, that it would ever be able to do that?" There's this thing called “directed evolution”. If I modulate the DNA, I can push it in this direction, but I don't understand why it happens. Evolution does it and it takes like five postdocs to be able to get there. And I was like, "All right, that sounds painful." But what it told me is there was an underlying relationship between modulating the piece of DNA that encodes the protein and the function of the protein. If you understood that relationship, you could skip the four years of postdoc and then directed evolution and have a machine tell you what piece of DNA you need to synthesize that will give you the functional protein you want.
That was the underlying insight that led to us starting to explore the idea that you could do. What we were calling “generative biology”, was that you could generate a piece of DNA sequence that would give you encode for any kind of protein that you wanted for any kind of function, and we started showing just examples of this. The first experiment that we did was on GFP. We showed that by learning on all of the GFP sequences that exist today, we could generate using machine learning, no protein structures, no previous information, we could generate that were 50 times brighter than anything that had been seen before.
That was a toy problem, but it was the first example that we were able to show that machine learning could engineer a protein.
Evan: At that stage, it makes me wonder—was there a group of people saying, 'Oh my God, you should do that, I believe it’s going to happen'? Or what percentage believed in it versus those who thought, 'That’s impossible'? And there might be a different group within Flagship, there might be more groups... Is there a different personality trait in that group, because you're building business all the time, versus maybe you have friends in the PhD and other parts of your life that are saying, "Molly, are you crazy? Why are you wasting your time with that? It's never going to work!" So, what was the balance and how did you fight that or go against it?
Molly: I would say there were a few labs that were starting to see signs of this throughout academic circles, and there were major protein engineering labs that were still in the biophysics realms and believed the biophysics was going to be the way and that was going to be how it happened. Most of the industry did not believe this was going to happen when we started. I remember having a conversation with... I won't name the company. I remember wanting to strike a partnership with them, be like, "Can we have just a little bit of data and then you can have rights to a lot of downstream potential opportunity?" They looked at us and laughed me out of the room.
Evan: It reminds me, I had a meeting at Kodak in 1997 when I was trying to get them to partner with us to build a broadband photo-sharing website. They said, "Why do we need an Internet imaging strategy?" And obviously, the rest is history. It's amazingly challenging. That's how elite thought businesses are created.
Molly: For sure, and within Flagship, we always take leaps. We're always thinking about the things that could be, and so we're oftentimes taught to suspend disbelief in these moments. While everyone was suspending disbelief, there was also still this kind of undertone of, "Okay, but we've been doing it this way for so long." It was interesting to build in that context, and it was a fun place to do it. I don't think there would've been any place that would've allowed us to build as big a vision as we did around it.
Evan: That's one of the things we love! We invest pre-revenue, pre-product and about 30% of the time we give a term sheet pre-incorporation, and your point about when people tell us, "Hey, it's going to be different. We're going to do it a different way in the future," and we do due diligence and reference checks on that opportunity with the legacy companies and they say, "No, you're crazy! It's never going to work. It's physically technically impossible." I was uncomfortable for our first five years of investing with that, but now we only invest when we hear that. When I say “It makes sense”, I get worried we're too late, and so it sounds similar. Let's jump to another topic – the creation of next-generation materials with AI. Can you give the audience a couple of examples of what spaces or sectors you're excited about and why now?
Molly: I can't think of a bigger problem than climate change/sustainability to not just the future health of our planet, but the future health of people. This all kind of started from that of, “What can we do to make an impact there?” I started looking at it from a biology lens because that's what Flagship primarily does. How do you use biology to change the world? Every time I looked at it, I recognized that we're already doing many of the things that we can do in agriculture, which is the biggest area that affects climate change. Indigo, Inari, and some other companies that we founded are already solving some of the big climate change challenges using agriculture as a muse.
I kept thinking that this is an important problem, but is biology the right tool? I kept coming to the answer that for things like carbon capture, again, outside of agriculture, I don't know that biology is the right tool for us to build better batteries or battery storage or get to a green hydrogen economy or whatever it is that we think is going to be the solutions to the problems we face.
I realized that the types of transformations we've seen in the protein space and in biology with generative AI don't exist, and the connections between labs and experimental labs and AI scientists don't exist. They don't talk, and so my question was, “Could we take some of the lessons we've learned from building Generate, some of the types of technologies we've built from building Generate, and build a similar type of organization and company for materials? Could we do generative AI for materials and integrate data and computation in such a way that you can develop differentiated products?” That was the origin of why we started diving into materials.
Evan: It seems like it's still early days in this next initiative, so we'll leave it at that. We have spoken about your views on the evolution of biology and material science from the past to the future. How will AI impact the historic scientific method?
Molly: If we think about how science looks today, the scientific method has been around for centuries, but it's this idea that we generate hypotheses, we go out in the world, we test those hypotheses, and then we update our mental model of how the world works. This is something that happens incredibly distributed within individual scientists' brains, and the only way that we communicate those updates to our mental model is through scientific publications, conferences, talks like this, whatever it is, that's our method for updating this distributed network of science within all of our brains. Is there a way in which we can do this differently?
Is there a way in which AI can come up with new scientific hypotheses and beyond that, go into the lab and test those hypotheses?
That's what we're thinking about. How do you think about transforming generative experimentation and the scientific method in a way that allows you to create a centralized mental model of science in ways that aren't possible today?
Evan: it's all about the papers that are now being published so quickly with so many variations, that it's even harder to keep up to date on everything. It seems like that should all be replaced. The question is, does that then commoditize or democratize in a positive way or commoditize in a negative way, the creation of science? I get asked this all the time as far as AI. There are a lot of things I don't want to do that I'd love AI to help me do 80% of my day, so I can focus the 20% on the strategy, the creativity and the human part. But is that similar in your field?
Molly: I agree with this. I've been spending a lot of time thinking about this in the last year or so. What is the world going to look like when AI is as powerful as people are predicting it will be? It's not a world where we aren't going to have creative things for humans to do. We're always going to have creative things for humans to do and we're going to change it. I have a prediction that everybody has no idea how many mundane things we do daily. If you weren't doing those things, you would be so much better at the things that you're uniquely capable of doing, and there's never going to be a world or I don't predict there's a world in which AI does all of the things we are going to do just because the limitations with resources, limitations with access.
There's going to be a human component to the future of science. There are going to be human components to the future of our society.
Evan: People worry about, "Hey, it's going to replace us." It's not going to replace us, but there are a lot of things I'd like it to replace. In your work, what are a couple of things that you're like, "The bane of my existence, I don't want to do that anymore. I want AI to do it." What would that be?
Molly: There are so many things like going to go toward recency biases because this is what I was doing today. I was reviewing a large contract for one of our organizations, reading the MSA and the statement of work and making sure that everything made sense. And I'm like, "Why am I doing this? Why can't I just upload this?" I ended up doing that, uploading this to ChatGPT and just having it answer all my questions. I didn't have to find it, and it worked to a certain degree. It could be better.
Evan: You probably have to validate the answers still, but it might help the process of it.
Molly: It helps the process. That was recency bias, but I'd say the things that I hope it will be able to do is to even tell me what the next experiment is that I might be able to do to test my hypotheses or scientists are constantly optimizing protocols, and this is something that has oftentimes have hundreds of parameters that you don't even know that you're making these decisions, but if a computer understood all those parameters, they could make decisions in ways that humans can't. Those are the types of things that we'll see happen.
Evan: In one-word answers - what is the best and least best personality trait for entrepreneurs?
Molly: So, the best is a growth mindset, worst is political.
Evan: What is the best and least best personality trait for PhDs?
Molly: Creativity is best and worst is linear.
Evan: What visual technologies are you most excited about in the next 20 years that relate to your work and your career or in general as a human?
Molly: AI having the potential to see is going to be the unlock to allow AI to go from the bits in our computer to the physical world. While I'm super interested in all of the structural graph neural network technologies that are going to exist for science, that's well proven today. What I don't think is proven yet is how computers seeing is going to change our physical world.
Evan: I love it! I didn't even give those words to you, but it validates our thesis! Hopefully, we’ll have more opportunities to collaborate in the future. I can't wait for that vision to come through!
Hope you enjoyed this fireside chat as much as we did. Check out other sessions too!
Here’s what Dr. Gibson said about our 10th Annual LDV Vision Summit: “Thanks for the invitation to join this year’s LDV Vision Summit! I had a great time discussing how the advancement of AI and integration with the lab are transforming science broadly – from biology to generating essential medicines to inorganic materials solving critical challenges in climate change. I’m most excited by the potential to marry AI, visual technologies, and robotics to transform the integration of AI with the physical world of science.”