Visual Tech Advancements in Life Sciences & Biotech Paving the Way for Longer and Healthier Lives
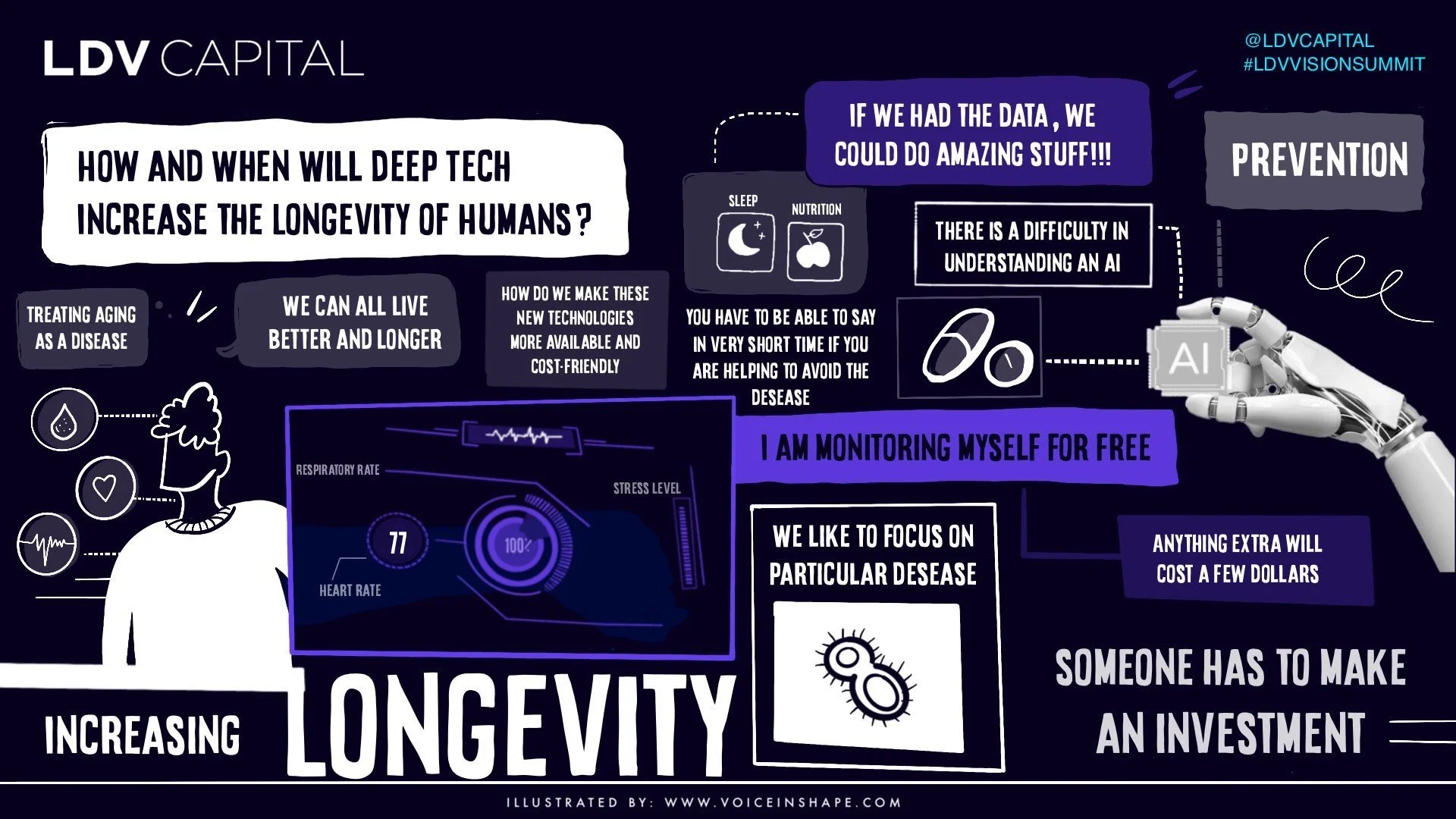
/As the biotech industry heavily relies on data filtering, analysis, and sharing, visual technologies play a critical role in detecting and imaging biological systems. This paves the way for the future of life sciences tools, enabling next-generation therapeutics, biomanufacturing, and longevity. At our 10th Annual LDV Vision Summit earlier this year, a panel of experts delved into these topics and explored various critical aspects shaping the future of biotechnology.
These leaders are pushing the boundaries of overcoming complex genetic diseases, improving health span, and providing actionable insights into environmental impacts on health.
Dr. Laura Smoliar, the Executive Director of the Taiwan Science & Technology Hub @Stanford. She is one of our LDV Capital Experts. In 2005, Laura founded Mobius Photonics, which developed high-power fiber-based UV and green lasers and was acquired by IPG Photonics. In 2016, Smoliar co-founded the Berkeley Catalyst Fund, where she made several early-stage biotech investments.
Neil Inala, CEO at Nobias Therapeutics, an AI-first therapeutics company. Nobias has recently completed a phase 2 clinical trial for treating symptoms of anxiety, inattention and autism in children with 22q11 deletion syndrome. Neil has been involved with software & systems development for three decades, including over 10 years at Google.
Dr. Olga Troyanskaya, Director at Princeton Precision Health, Professor at Princeton University and Deputy Director for Genomics at the Flatiron Institute of the Simons Foundation. She is a fellow of the ACM and among her honors and awards are the 2014 Ira Herskowitz Award from the Genetic Society of America, the 2011 Overton Prize, from the International Society of Computational Biology, and the 2011 Blavatnik Award For Young Scientists (Finalist Award).
Dr. Elizabeth Neumann, an Assistant Professor at the University of California, Davis. Her lab focuses on understanding the molecular and cellular architecture behind neurological diseases. The research is highly interdisciplinary and involves developing analytical tools and multimodal imaging methods for understanding complex biological phenomena.
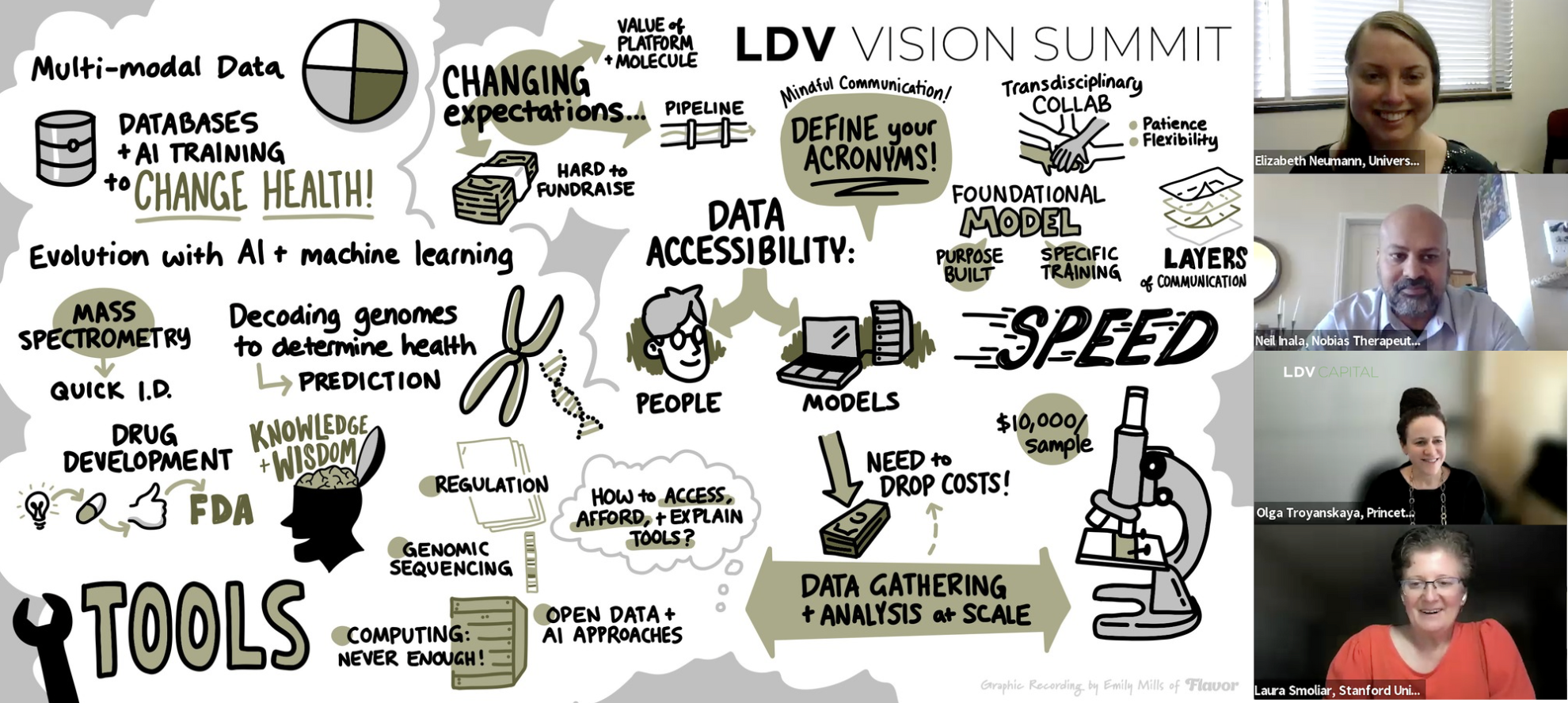
The explosion of AI coupled with visual technology is a huge unlock for the life sciences. Training models on multimodal data is powerful and enabling. Re/Watch this panel discussion or check out our shortened and lightly edited transcript below.
Laura: Why is spatial biology garnering so much attention now?
Elizabeth: If you think about mass spectrometry, we're detecting tens of thousands of features in fractions of a second. So we can probe 30 cells a second. As you can imagine, that's tens of thousands of things we know and hundreds of thousands of chemicals that we're detecting that we don't know. Machine learning allows us to quickly parse through these different molecules that we're detecting and then see how they are intrinsic to either a cell type or a disease state. It allows us to look at these large patient cohorts quickly, running the gambit from individual cells to the full organ system. Our ability to do this and how much data we can handle has evolved since I was a graduate student. It's quite impressive!
Laura: How has the evolution of visual technology and its intersection with machine learning and AI impacted the work in your field in the last 1-3 years, and what do you see as problems that are feasible to tackle now that weren't possible before?
Olga: My group builds AI frameworks to decode genomes and understand cellular specificity and how your genome is related to health to be able to detangle the biology underlying complex diseases. For us, the AI revolution has been transformative because the emergence of deep learning approaches has enabled us to pioneer the use of these models to study regulatory genomes to be able to computationally predict the effect of any mutation. This is especially critical for mutations outside of genes, which is 98% of your genome that controls how genes are turned on and off, which in term affects your cells, organs, and of course finally your health or treatment from disease. Of course, these don't act in a vacuum. They act in specific cellular and tissue and disease contexts. The explosion of omic and spatial data is critical to be able to understand the effects of these mutations and your genome sequence on your health and disease. These complex molecular-level changes are captured via diverse omics techniques, including specific clinical treatment and environmental contexts. AI frameworks that integrate such signals can enable us to have a systems-level molecular view of human health and disease.
Neil: Our general and biggest problem is that we have an absolute wealth of information about biology, but it’s locked away in databases without a true conceptual understanding. We need to figure out how to turn this huge variety of information, often giant disconnected databases, into something useful and actionable for drug development. There's a secondary problem of how you develop a drug, a molecule that will make it through this sort of series of gauntlets, of course, just being effective, getting through the liver, getting through to the point of application in the cell or at the target site, and then, of course, getting it through the FDA and showing efficacy. But how can we know more about what's out there? How can we turn these disconnected databases into something useful? We want to transform this stuff from data to information to knowledge to wisdom. That comes from developing greater tools for understanding.
Whereas now with a lot of the recent discoveries, particularly in the space of semantic and instance segmentation and visual processing, and also with Generative AI, I think we've seen a much greater expansion of the tools that exist that might enable us to understand and connect things a little bit more at a conceptual level. And once we understand things, we can pull together sort of disparate otherwise disconnected concepts, we have a much greater chance of building something for drug development, for example, that will be a useful molecule rather than trying a whole bunch of random things and hoping that they work.
Laura: What are the most exciting developments on the horizon from your vantage points and what tools and or resources are most enabling for you? Is access a challenge, to get these tools in academia or a startup as opposed to the large tech companies? And how are you addressing that?
Olga: The explosion in the AI space and of course the explosion of multi-omics data is exciting! The most exciting promise is the genome-based integrative approaches that can change how we do precision health. Being able to leverage such AI frameworks to identify the genomic signals that can be combined both for diagnosis and for treatment guidance can enable the future of treatments that are coming on the horizon.
It does rely on a lot of compute, which is always a challenge, but especially if you talk about startups and smaller companies and universities, this is a major limiting factor.
I'm most excited that all of this tech and data revolution can enable us to start looking at treatments that don't just modify proteins but are targeting regulation, targeting how an expression is regulated and which version of the protein, or isoform, is being produced. There's enormous promise to be able to reach the previously undruggable targets, as well as potentially provide more precise control for these treatments and minimize side effects. That relies on access to data, having data as open as possible. That's another side of this in terms of the resources as well as the continuous ability to develop novel AI approaches and be able to run them with enough compute.
Neil: The most exciting things that are coming down the pipeline are probably things that stem from a greater understanding of tools to organize and develop information and incorporate that into something useful for us.
There's no general answer for that, but specific things include Generative AI, for example, which most of you probably know from Stable Diffusion, Midjourney, and things like that where maybe you type in a text that describes an image. The general concept behind that is the exploration of noise generation from a semantic space. We use that concept for a variety of things. For example, for generating conformers, which are used in efficient sampling and molecular modeling. Which kinds of molecular setups or conformers can interact with a protein? But more generally, data gathering and analysis at scale is something growing. We see that in certain startups like Recursion for example. They're doing a lot of this visually with conformal microscopy. The ability to do this using robotics and imaging tools will generate huge streams of new data, which will be incredibly useful. In addition to that, things like new sequencing tools, but there's a challenge to that as well, which is that the costs are still high.
As a startup, we have limited funds. Of course, most of that funding needs to go to paying salaries. We pay for compute costs. Some programs help like the NVIDIA Inception program for example, which can dramatically reduce the cost of renting or buying hardware and getting cloud access. But for things like sequencing, we still need to bring down costs, even though costs have dropped to the point where they're a few hundred dollars sometimes.
Some things may still be challenging, like single-cell sequencing, ATAC-Seq, and proteomics, where we need to bring costs down by another order of magnitude to do this at scale across specific patient populations.
Laura: Olga, we talked a little bit about foundational and purpose-built models: will we ever get our hands around biology or is it just too complicated?
Olga: I think of foundation models as any model that takes in large amounts of more general data that then can be fine-tuned for specific questions, which of course is extremely useful for biology. For example, we have quite a bit of data on what genes are turned on and off in the brain. But if you are thinking about studying neurodevelopmental disorders, you need data for early fetal stages as well as early infancy, and we have very little data there. A foundation model could be a powerful way to do this.
It’s important to consider whether we will soon have a foundation model trained for all of biology. If we define all of biology as something that will help students learn biology, sure, that's great. But if we're thinking about models that are going to help us in the precision health and precision medical context, I'm going to bet on purpose-built models in the near future, models that are going to be built specifically for medical clinical questions and perhaps even more specifically trained or at least fine-tuned for specific aspects of the field. We need to realize that there's a lot we know about biology, it's a complex field. Foundation models are going to be transformative, and purpose-built models for health is where I expect most of the impact in the field.
Laura: Neil, I wanted to ask you about, as a company, how we used to talk about are you a platform company or a therapeutics company? And you had some interesting insights on how investor expectations are changing in this area.
Neil: There's a little bit of a ping-ponging back and forth between what's the value of a platform versus what's the value of individual molecules and therapeutics.
I think that it will always be important in biotech and pharma for you to have molecules and treatments that are going to end up going to patients, but then the concept of how do you continue to develop those and how do you create a pipeline, it varies somewhat based on people's time horizon and that time horizon, as we know it shifts based on the yield curve. As people become more shortsighted, maybe platforms become less important. As people become a little bit more thinking long-term, then platforms become something that they think about.
We thought from the beginning we needed to have that pipeline and we needed to have therapeutics, which is one reason we became a clinical-stage company. We have two compounds, one of which I think I may have mentioned. We recently reported interesting results in a rare disease population, neuropsychiatric results. But the fact that we have these therapeutics available as sort of validation of our platform has also made it hard to raise capital because people are looking at the therapeutic only and then they're not attributing major value to the platform. Perhaps as the economy gets better and people start to shift again to thinking about that platform, then maybe that will shift. It's a balancing act and it requires a lot of dynamic insight into the market and figuring out where things are going.
Laura: Let’s talk about cross-disciplinary collaboration. It's always a challenge for people to speak different languages in computer science and biology, so I'd love to hear how you deal with that.
Olga: This is the age of collaborative and integrative frameworks. If we're talking about AI and medicine, there is not much you can do in your corner office by yourself. That’s why my group is highly interdisciplinary. We have computer scientists and computational biologists. In everything we do, we collaborate with clinicians and experimentalists. We have an experimentalist in the group.
Beyond individual groups, at Princeton, we launched a Princeton Precision Health Initiative where we aim to change health policy and clinical practice through AI and data science. I call Princeton Precision Health ‘transdisciplinary’ because it forces groups like mine that are already often interdisciplinary to integrate far beyond our usual sphere - with sociologists, economics, ethicists, and policy researchers. After all, we're trying to go from genomic and molecular health signals to environmental data, epidemiological level, socioeconomic and lifestyle factors, and beyond. We must implement these models and studies in an explicit ethical framework.
To effectively manage such broad collaborations, you have to be patient and you have to listen, and you have to identify, and realize that each of us has to be flexible. It's not just about understanding other domains - to make major changes, we each have to be a bit more fluid and dynamic in our research directions and how we work both long-term and day-to-day - that’s how we can make the most transformative impact.
Neil: Our engineers look at things from an engineering point of view, and they're brilliant, but we also have to connect that to clinical development. Our engineers have specializations ranging from things like particle physics to molecular modeling to pathway biology, with AI knowledge overlaid on top of that. On the other side, we have experts in clinical development who know the ins and outs of turning a new chemical entity into a drug that's useful for patients by passing FDA regulatory approval, manufacturing it, and getting it commercialized. Connecting the engineering side and the clinical side is challenging. We have to do that across many different levels and domains, and that requires a huge investment in communication.
“The summit enabled us to discuss critical aspects of AI development and impact in the precision health space. I am especially excited about the promise of purpose-built AI models for biomedical space that can connect heterogeneous genomic and molecular data with clinical outcomes, enabling precise diagnosis, treatment, and accelerating next-generation drug development." - Dr. Olga Troyanskaya, Director at Princeton Precision Health, Professor at Princeton University and Deputy Director for Genomics at the Flatiron Institute of the Simons Foundation
"I really enjoyed being on the LDV Vision panel discussing how AI and visual tech can advance the life sciences and biotech - these kinds of cross-disciplinary discussions create fertile opportunities for new ways of thinking about convolutional and spatial processes. The other keynotes were compelling as well, and I was struck by the wide-ranging concepts covered in all the different panels. Thanks to the Summit team for putting together such interesting groups!" - Neil Inala, CEO at Nobias Therapeutics
“This was my first Annual LDV Vision Summit and I hope I'm invited back! It was an incredible opportunity to hear from leaders in the field from a variety of backgrounds. Spatial biology is hugely important and I'm excited to see what the future holds." - Dr. Elizabeth Neumann, an Assistant Professor at the University of California, Davis